We can all acknowledge that the title of this post is not that funny-at least, not to anyone other than medical device regulatory nerds, such as the author of this post. What no one should find funny is how CDRH seems to be approaching Breakthrough devices of late, setting clinical study expectations so high that these innovative, novel, life-saving products may never get to the other side of the door, regardless of how much they knock.
CDRH takes great pride in stating that it is committed to innovation. In fact, it has a whole page dedicated to “CDRH Innovation,” which states that CDRH “is committed to advancing public health by helping to bring innovative technologies to market.” In particular, CDRH touts its Breakthrough Devices Program, which it advertises as a “program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. Manufacturers can expect increased interaction with the review team and prioritized review of the marketing submission.”
The reality, however, is quite different. The Breakthrough program started in 2015. Since that time, CDRH has granted 921 Breakthrough devices, and CBER has granted 12. This graph shows the number of granted Breakthrough Device designations by fiscal year.
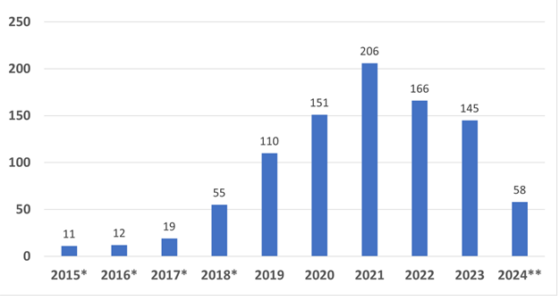
Of the 933 devices granted Breakthrough status, a whopping total of 95 have received marketing authorization (91 CDRH devices and four CBER devices). That is just barely over 10% in almost a decade. While there can be any number of reasons why so few have received authorization, if this were truly a program to benefit innovative products, it would stand to reason that more would have been successful in getting to market, especially given the volume granted designation in 2020-2021.
In advising clients who have received Breakthrough designation, one theme seems to be emerging, which we expect is responsible for at least some of the missing authorizations-CDRH’s clinical trial expectations, especially for low-prevalence conditions, can almost guarantee that most of these Breakthrough devices never see the light of day. CDRH seems unwilling to move away from a theoretical world in which money and time are abundant and enriching studies is a simple task. For certain indications or populations, a prospective study is simply infeasible-even a well-funded, large company is unlikely to enroll tens of thousands of study participants to properly power a study for a low-prevalence disease. Even for higher-prevalence conditions, CDRH’s recent feedback in pre-submission meetings shows just how far apart the review teams are from the reality of what it takes to run a robust clinical trial.
For many companies, start-ups in particular, a request for Breakthrough designation may be their first interaction with FDA. These requests are often based upon promising data from early feasibility studies, and the indication requested stems from the results of those small studies without considering what the requirements might be for a pivotal study to support the same indication.
If a company is successful in obtaining Breakthrough status, when it goes back to the FDA with a proposed pivotal study design, it may not like what it hears-that a study to support the Breakthrough indication will need to be larger and more complex than the company likely anticipated or is able to support. This then puts the company in the unenviable position of deciding whether to abandon its Breakthrough status to design a more palatable study for a different indication, or to negotiate study design with the Agency to support the Breakthrough indication. And while FDA repeatedly states that it does not design studies, the sponsors do, the hard truth is that only FDA can ultimately deem a study adequate to open the door to allow the Breakthrough device to cross the threshold into the marketplace.
So what can be done? There is no reason for the Breakthrough to be the first interaction-it only has to be submitted to the Agency before submitting the marketing application. Therefore, while there is no guaranteed path to success, one suggestion is that before going to FDA with a Breakthrough designation request, companies should submit a standard pre-submission with proposed indications and clinical trial design. If a company takes this approach before submitting a Breakthrough request, the company can gain alignment with FDA on what its expectations will be for a study to support the indication desired by the company, before getting a Breakthrough indication only to learn that FDA’s clinical trial expectations are impractical.
CDRH should take a close look at the Breakthrough designations granted to date, how many sponsors with Breakthrough devices submitted marketing applications that were not ultimately granted, and how many came in with pre-submissions with requests for feedback on clinical trial design that ultimately never submitted a marketing application. If CDRH truly wants to be a hub of innovation, it needs to find ways to work with companies to facilitate market access, rather than slamming the door in their face.

